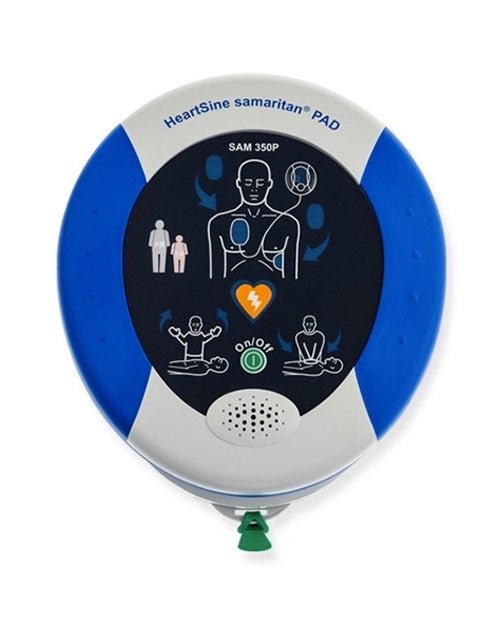
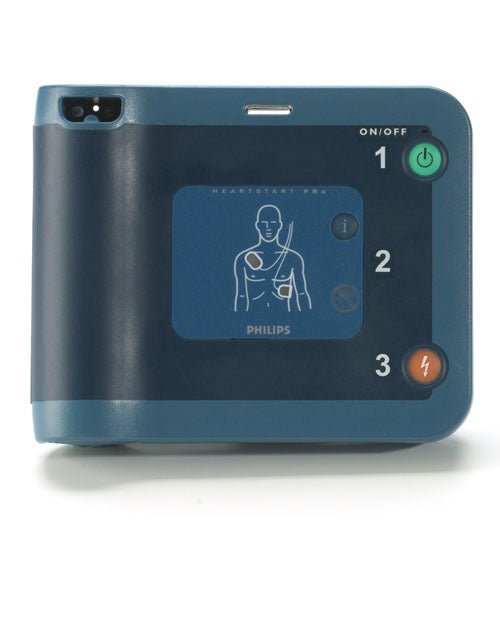
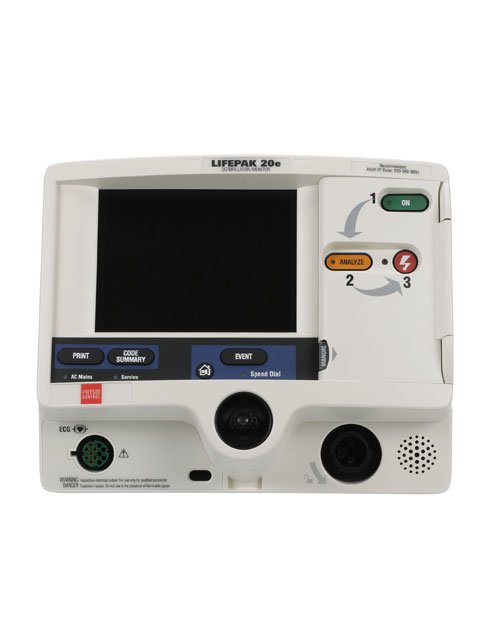
Allow AED.US to Help You Stay in Compliance
WHAT IS A PRE-MARKET APPROVAL OR PMA?
Premarket approval (PMA) is the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices. Class III devices are those that support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury.
WHEN IS A PMA REQUIRED?
The FDA published a final order in 2015 requiring premarket approval applications for new and existing AEDs and accessories. All AEDs must go through the FDA’s PMA process in order to be approved for sale in the US.
ARE MY AEDs AFFECTED?
In 2019, the FDA notified AED manufacturers who did not submit a PMA application for their AEDs, that they are no longer allowed to market their AED. It was also stated that by February 3, 2020*, manufacturers of accessories (such as batteries, pad electrodes, adapters and hardware) for FDA-approved AEDs, are required to file a premarket approval application. If a PMA is not filed by then, the manufacturer must cease marketing and can no longer support these accessories effective February 2022. This deadline also applies to accessories intended for AEDs that are not FDA-approved.
WHAT SHOULD I DO?
To ensure your device is approved by the FDA, check for your model on the table below. If it is not on the list, contact us today for more information on how we can assist you in transitioning to a premarket approved device and how you can earn up to $625 through our trade-in program.
*FDA deadline moved to February 3, 2022: "The FDA has since issued a guidance to revise its compliance policy regarding the deadline for filing PMAs for these necessary AED accessories, announcing that the FDA does not intend to enforce compliance with the PMA submission requirement for these necessary accessories until February 3, 2022. This revised compliance policy allows facilities additional time, particularly during the COVID-19 pandemic, to procure FDA-approved AEDs and manufacturers to file the required PMA for necessary accessories, respectively."